Recording collected clinical trial data accurately into the database
Transform your clinical data into actionable insights.
Reduce errors,risks, and costs by ensuring quality and accuracy of
clinical trial data for faster regulatory submissions

The success of a clinical trial depends on collecting and managing research data in line with regulatory standards for statistical analysis. Our Clinical Data Management (CDM) services ensure that your data meets regulatory quality assurance mandates, while also accelerating the development of pharma products.
At Inductive Quotient, our team of expert professionals comes with a deep understanding of the needs and challenges of clinical research. We design a customized CDM solution after learning your specific requirements to ensure high-grade data quality and precision.


Our approach begins with building a stack of tools and techniques to collect clinical data based on the study protocol, followed by a data validation process assuring compliance with regulations like ICH-GCP, CDASH, and other regulatory requirements. This clean data is sent for statistical analytics and programming to extract insights.
Recording collected clinical trial data accurately into the database
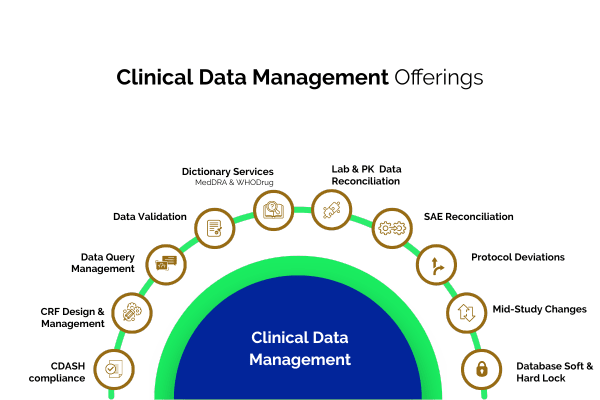
Creating Case Report Forms (CRFs) tailored to the study protocol for structured data collection.
Checking data for accuracy, consistency, and completeness to meet regulatory standards
Building a secure, efficient, and compliant database for storing clinical trial data.
Recording collected clinical trial data accurately into the database
Creating Case Report Forms (CRFs) tailored to the study protocol for structured data collection.
Checking data for accuracy, consistency, and completeness to meet regulatory standards
Building a secure, efficient, and compliant database for storing clinical trial data.
Converting medical terms into standardized codes (e.g., MedDRA, WHO-DDE) for uniform reporting
Identifying and resolving data discrepancies through communication with study sites
Matching and verifying data across different sources to ensure accuracy
Comparing Serious Adverse Event (SAE) reports with clinical data to maintain consistency
At IQA, we are uncompromising when it comes to data security and governance. We employ cutting-edge technology and security guidelines to safeguard your clinical data throughout clinical trial research.
Collaborate with us to resolute data quality aligning with the regulations thereby affirming the success of your clinical trials